The latest development of new drug development for macular degeneration in May 2018
June 05, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Age-related macular degeneration (AMD) is a disease that causes blurred or lost central vision. AMD is a common eye disease in people over the age of 50 and is the leading cause of vision loss. It causes a small spot near the center of the retina - the macula causes damage. The macular is made up of millions of light-sensitive cells that provide clear central vision. It is the most sensitive part of the retina, located at the back of the eye. When the macula is damaged, the center of the field of view may appear blurred, distorted, or dark. AMD can be divided into early, middle and late categories. The late stage is further divided into "dry" and "wet", of which dry macular degeneration accounts for 90%.
"Wet" macular degeneration (Wet AMD) is also known as neovascularization or exudative AMD. In this advanced AMD, abnormal blood vessels (called choroidal neovascularities) grow under the retina and the macula. These abnormal blood vessels are very fragile and can cause leakage of blood and proteins. Bleeding, leakage, and scarring of blood vessels can eventually cause irreversible damage to photoreceptors, and if left untreated, vision will drop rapidly. Vascular endothelial growth factor (VEGF) stimulates the proliferation of abnormal blood vessels in the retina. Therefore, wet macular degeneration can slow the progression of the disease by intraocular injection of anti-VEGF drugs.

â–² Vision of patients with normal vision and macular degeneration (Source: By National Eye Institute, National Institutes of Health [Public domain], via Wikimedia Commons)
According to the American Academy of Ophthalmology, the number of patients with new macular degeneration in the world exceeds 1 million per year. It is estimated that by 2020, AMD patients in Western countries are expected to reach 25 million, becoming the main culprit in blindness in the elderly. The data show that there are about 300,000 new age-related age-related macular degeneration patients in China at this stage. With the full arrival of an aging society and the direct impact of the blue light on the mobile phone's LED screen on the eyes, the degree of damage in the macular area of ​​the eye has accelerated. AMD has become the third most blind eye disease, and it is listed as a disease field in the world to explore and research. one. In 2013, China approved the national first-class innovative drug Conbercept, which has become a new milestone in the development of China's AMD drug field. On May 10 this year, Bayer's Ai Liya® (Abersip Intraocular Injectable Solution) was obtained by the State Drug Administration for the treatment of adult neovascular (wet) age-related macular degeneration (nAMD).
This article takes stock of the latest clinical development pipeline for macular degeneration in May 2018. We calculated clinical trial data for clinical stage 1, phase 2, and phase 3 macular degeneration drugs registered on clinicaltrials.gov. Included in the statistics are trials that are being recruited, recruited, and issued recruitment notices, and do not include trials that have been completed, terminated, unknown, withdrawn, and suspended.
Progress in clinical research and development of new drugs for macular degeneration
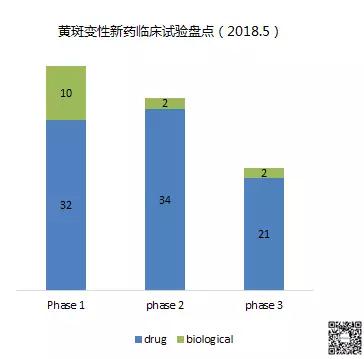
As of May 31, 2018, there were 101 clinical trials of new drugs in the field of macular degeneration, with phases 1 and 2 predominating. These include: 42 trials in Phase 1, including 32 small molecule drugs and 10 biopharmaceuticals; 36 trials in Phase 2, including 34 small molecule drugs and 2 biopharmaceuticals; Twenty-three trials, including 21 small molecule drugs and 2 biological drugs (note: some drugs may be tested simultaneously).
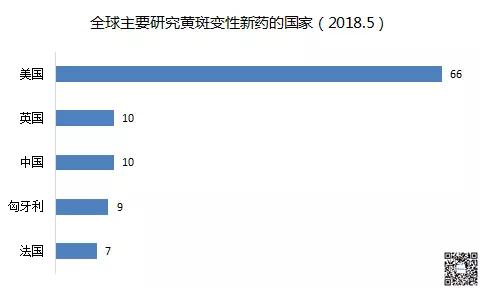
The clinical trials of new drugs for macular degeneration are concentrated in the United States. According to the location of the research institutions of clinical trials, the top 5 countries are: 66 trials in the United States, 10 in China, 10 in the UK, 9 in Hungary, and 7 in France. : There may be a multicenter clinical trial).
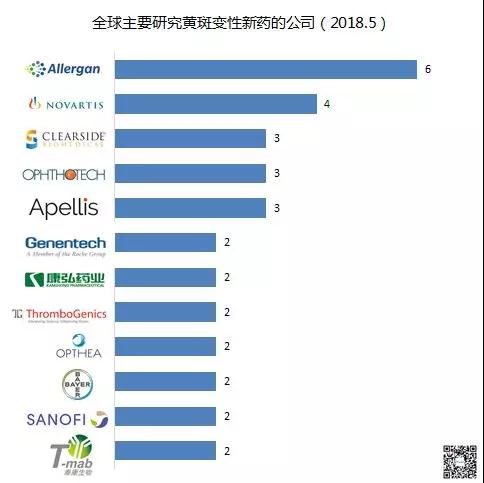
The world's leading companies investigating new products for macular degeneration are: Allergan 6 trials, 4 Novartis, 3 Apellis, 3 Ophthotech, 3 Clearear, 2 Chengdong Kanghong, 2 Jiang Su-Mab, 2 Sanofi, 2 Genentech 2 items of Bayer, 2 items of Opthea, 2 items of ThromboGenics.
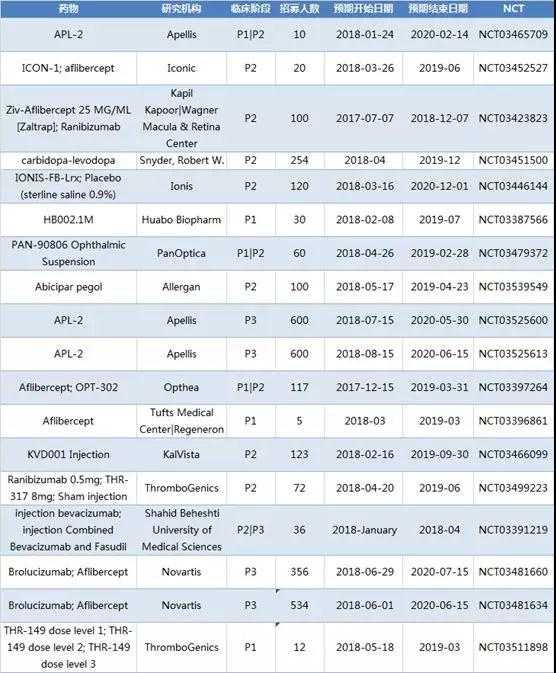
New clinical trial of new macular degeneration in 2018
In 2018, 18 new clinical trials of macular degeneration were added. An average of 62 subjects were recruited in 3 1/2 trials, 16 subjects were recruited in 3 phase 1 trials, 36 subjects were recruited in 1 phase 2/3 trial, and an average of 7 trials in phase 2 trials were recruited. Of the 113 subjects, 4 of the 3 trials enrolled an average of 523 subjects.
Spinal fixation system consists of posterior spinal fixation system,minimally invasive spinal system,posterior cervical fixation system,anterior cervical plate system,laminar shapping plate system and interbody fusion cage system.
Cervical and lumbar segmental implants are the most common types of spinal internal fixation, which can be roughly divided into anterior and posterior internal fixation according to their fixed positions and surgical approaches.
The anterior cervical vertebra is mostly fixed with locking plates and fixed screws,what are mostly made of titanium alloy materials; The posterior approach was fixed using the pedicle screw and rod system.
In some cases of spinal vertebrae bone defect, it is also necessary to implant titanium cage or PEEK cages to promote bone fusion of adjacent vertebrae. The titanium mesh cage refers to a cage shaped container made of titanium alloy material, which is loaded with autologous or allogeneic bone and placed in the spinal vertebrae bone defect, which not only serves as a strength support but also plays a role in bone fusion.
spinal fracture,Pedicle Screws,Spinal Implant,Spine Implants
Jiangsu Aomed Ortho Medical Technology Co.,Ltd , https://www.aomedortho.com